Solution Dilution Calculator app for iPhone and iPad
Developer: Nitrio
First release : 28 Feb 2018
App size: 7.73 Mb
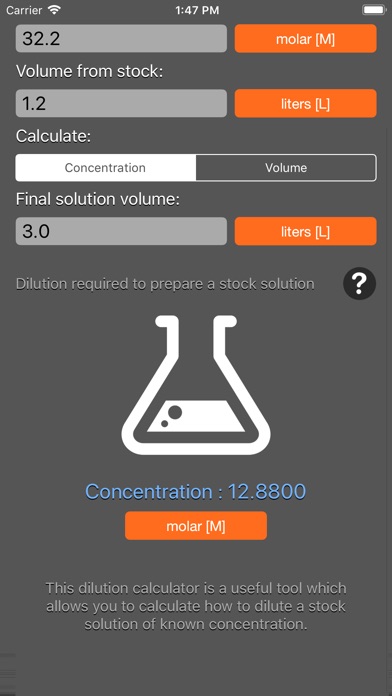
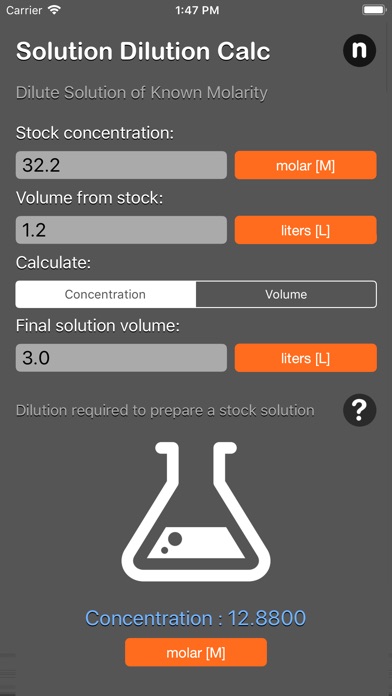
The solution dilution calculator calculates the volume of stock concentrate to add to achieve a specified volume and concentration. The calculator uses the formula C1V1 = C2V2 where "1" represents the concentrated conditions (i.e. stock solution Molarity and volume) and "2" represents the diluted conditions (i.e. desired volume and Molarity).
Meant to be used in both the teaching and research laboratory, this calculator can be utilized to perform dilution calculations when working with solutions having mass per volume (i.e., mass over volume) or weight per volume (i.e., weight over volume) concentration units.
The dilution calculator is based on the following equation:
Concentration(start) x Volume(start) = Concentration(final) x Volume(final)
This equation is commonly abbreviated as: C1V1 = C2V2
C1 is the concentration of the stock solution.
V1 is the volume to be removed (i.e., aliquoted) from the concentrated stock solution.
C2 is the final concentration of the diluted solution.
V2 is the final volume of the diluted solution. This is the volume that results after V1 from the stock solution has been diluted with diluent to achieve a total diluted volume of V2.
An example of a dilution calculation:
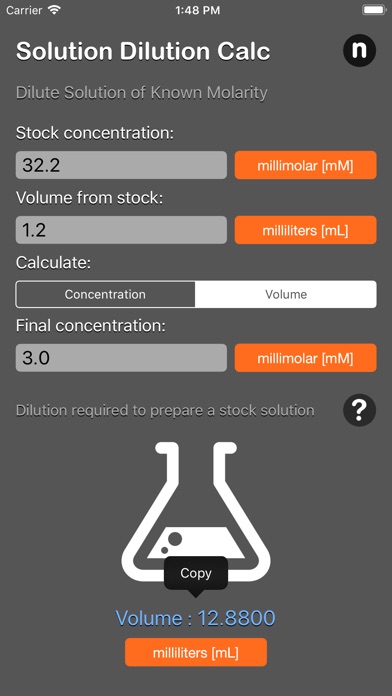
What volume of a given 10 mM stock solution is required to make 20ml of a 50 μM solution?
Using the equation C1V1 = C2V2, where C1=10 mM, C2=50 μM, V2=20 ml and V1 is the unknown:
- Enter 10 into the Concentration (start) box and select the correct unit (millimolar)
- Enter 50 into the Concentration (final) box and select the correct unit (micromolar)
- Enter 20 into the Volume (final) box and select the correct unit (milliliter)
- The answer of 100 microliter (0.1 ml) will appears automatically
*Thanks for your support, stay tune for more update to come.